Professional Manufacturer of Biomagnetic Beads

Color-Shifting Magnetic Fluids: The Mechanism Behind the Hue
If magnetic fluids were a “versatile fashion icon,” their wardrobe would hold top-secret scientific mysteries—today adorned in elegant primrose yellow, tomorrow switched to classic pitch black. But hold on! This isn’t nanoparticles playing dress-up; it’s a precision-controlled show of physical chemistry! Let’s unveil the secrets behind this “color magic.”
Chapter 1: Decoding the “Yellow & Black” of Magnetic Fluids: The Color Code of the Nano Realm
1、Black: The Classic “Safe Choice”
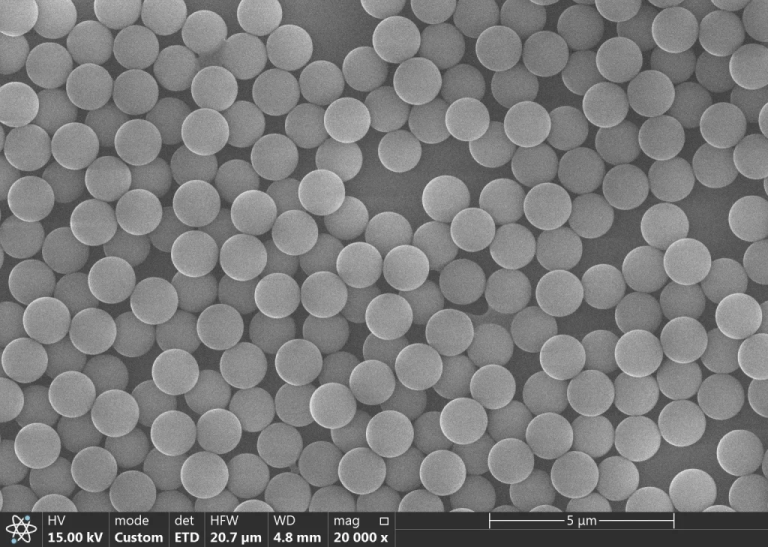
Starring as Itself: Pristine magnetite (Fe₃O₄) nanoparticles (~10 nm) are born with “dark skin.” They devour all wavelengths of visible light greedily, leaving you with nothing but profound black.
Agglomeration Bonus: When particles clump together (>100 nm) due to electrostatic imbalance, they trigger the “Mie scattering effect”—intensifying the blackness into a nano-scale black hole.
2、Yellow: The Limited-Edition “Haute Couture”
Surface Gilding: Through modification with sodium citrate, Fe³⁺ forms a monodentate complex [Feᴵᴵᴵ(H₂Cit)]⁻, which fiercely absorbs blue-violet light at 418 nm, reflecting a brilliant golden hue.
Scattering Assist: The innate talent of 10 nm particles as “blue light assassins” (Rayleigh scattering: I ∝ 1/λ⁴) teams up with citrate yellow to create a vibrant amber glow.
Chapter 2: Why Won’t Your Magnetic Fluid Turn “Yellow”? A Post-Mortem of Kitchen Disasters
Trying to replicate “Minion”-yellow fluid but ended up with a pot of sesame paste? How many of these mishaps did you commit?
| Disaster Cause | Scientific Principle | Outcome |
| pH Chosen as 8.5 | In alkaline conditions, NH₃ hijacks citrate binding sites (Fe³⁺-NH₃ β=10³³ >> Fe³⁺-Cit³⁻ 10²⁵) | Citrate: “No room for me!” |
| High-Temperature Overcooking | >50°C converts Fe₃O₄ to γ-Fe₂O₃ (4Fe₃O₄ + O₂ → 6γ-Fe₂O₃) + decomposes citrate | Magnet → charcoal, citrate → sludge |
| Oxidation Sabotage | Divalent iron (Fe²⁺) instantly oxidizes in air, slashing magnetism by 40% | Weak magnetic field and dark color→ “battle-scarred edition” |
Witty Tip: Want yellow magnetic fluid? Remember—don’t befriend alkalinity, don’t brother with heat!
Chapter 3: Step-by-Step Guide to “Yellow”: The Nano Chef’s Secret Recipe
Craving a magnetic “mojito” with strong magnetism (Ms >75 emu/g), suspension stability (Zeta < -40 mV), and radiant color (b* >35)? Three steps to perfection!
Formula Table
| Material | Role |
| Sodium citrate | Chief Dyer (locks Fe³⁺ via monodentate binding) |
| Ascorbic acid | Antioxidant Bodyguard (Fe²⁺ protection squad) |
| PEG-2000 | Suspension Coach (steric hindrance against clumping) |
| pH=5.5 buffer | Acidic Stage (H₂Cit⁻’s主场) |
Operating Guide (Enchanted Edition)
1、Cleanse the Black: Scrub Fe₃O₄ with pH=4.0 dilute HCl ×3 to remove oxidized “grime.”
2、Low-Temp Coloring: Add citrate + ascorbic acid to pH=5.5 buffer. Stir gently at 25°C for 45 min (1°C hotter = disaster!).
3、PEG Setting: Transfer to 35°C “warm room” under N₂. Age for 24 hours—too short? Yellow won’t mature!
Chapter 4: Why “Yellow”? The Golden Allure in the Commercial World
Black magnetic fluids are practical, but yellow ones are tech’s superstars!
Exhibition King: In science museums, yellow fluid dances into golden spikes under magnetic fields, outshining black’s “dull peaks.”
Biological Tag: Fluorescent-labeled yellow fluid becomes a “nano GPS” for cell tracking.
Anti-Counterfeiting Elite: Custom magneto-optical hues make forgers cry—”Impossible to replicate!”
Sneak Peek: Next up—rainbow magnetic fluid? Scientists already dye SiO₂-coated particles red/blue/green. The magnetic runway will light up the intersection of tech and art!
Closing Easter Egg:Next time you see yellow magnetic fluid shimmering gold in a magnetic field, applaud—it’s the scattering ballet of 10 nm particles, harmonized with the optical solo of citrate complexes, conducting a “nano-symphony” of pH and temperature precision!
Ultimate Advice: To master magnetic fluid colors? Remember: Acidity is the stage, low temperature the rhythm, citrate the conductor—or it will bow out in black!
Place your order without hesitation today!
Supplier
Shanghai Lingjun Biotechnology Co., Ltd. was established in 2016 which is a professional manufacturer of biomagnetic materials and nucleic acid extraction reagents.
We have rich experience in nucleic acid extraction and purification, protein purification, cell separation, chemiluminescence, and other technical fields.
Our products are widely used in many fields, such as medical testing, genetic testing, university research, genetic breeding, and so on. We not only provide products but also can undertake OEM, ODM, and other needs. If you have a related need, please feel free to contact us .