Professional Manufacturer of Biomagnetic Beads

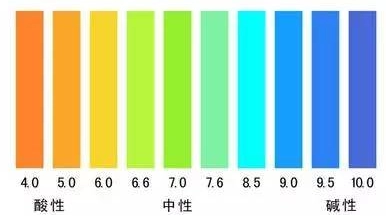
How much influence doespHhave on silica hydroxyl and carboxyl magnetic beads?
The influence of pH on the performance of Silica hydroxyl and carboxyl magnetic beads is extremely significant and operates through fundamentally different mechanisms. It directly affects their binding capacity, stability, and application effectiveness. Understanding these effects is crucial for the correct selection and use of magnetic beads.
Below is a detailed analysis:
1. Impact of pH on Silica hydroxyl (Si-OH) Magnetic Beads
Mechanism: Silica hydroxyl magnetic beads primarily rely on the negative charge generated by the deprotonation of surface Silica hydroxyl groups (-Si-OH → -Si-O⁻). Binding to negatively charged biomolecules (like the phosphate backbone of nucleic acids) occurs via a salt bridge (cation-mediated electrostatic attraction).
Effect of pH:
Low pH (<7 – Neutral):
Silica hydroxyl groups are predominantly protonated (-Si-OH), resulting in little to no surface negative charge (or even a positive charge at very low pH).
Very Low Binding Capacity: Insufficient negative charge prevents effective salt bridge formation with negatively charged nucleic acids (phosphate groups are also partially protonated, reducing their negative charge).
Bead aggregation may occur.
High pH (Alkaline, typically ~8-10):
Silica hydroxyl groups are extensively deprotonated (-Si-O⁻), leading to high surface negative charge density.
High Binding Capacity: In the presence of high concentrations of chaotropic salts, cations shield the electrostatic repulsion between the negatively charged nucleic acid phosphate backbone and the negatively charged bead surface. This allows tight binding via cation bridges (-Si-O⁻…M⁺…⁻O-P-).
Stability Risk: Excessively high pH (>10-11) significantly accelerates the dissolution of the silica matrix (especially at elevated temperatures), destroying bead structure, releasing silica ions, and potentially affecting downstream applications (e.g., PCR inhibition).
Optimal Range: Nucleic acid binding is typically performed in the pH 8.0 – 9.5 range (common binding buffers include Tris-EDTA or sodium acetate buffers with high chaotropic salt concentrations, pH-adjusted to this range). Elution usually occurs at lower pH (e.g., pH 7.5-8.0) or in low-salt buffers.
2. Impact of pH on Carboxyl (-COOH) Magnetic Beads
Mechanism: Carboxyl magnetic beads primarily rely on the protonated state of surface carboxyl groups (-COOH) to bind target molecules (like proteins, antibodies) via hydrophobic interactions and hydrogen bonding. Deprotonated carboxyl groups (-COO⁻) generate negative charge, which can be used for ion exchange or to create steric hindrance/hydrophilicity to reduce non-specific adsorption.
Effect of pH:
Low pH (Acidic, < Carboxyl pKa, typically <4.5-5.0):
Carboxyl groups are highly protonated (-COOH), making the surface electrically neutral or weakly negative.
Strong Hydrophobic Binding Capacity: Protonated carboxyl groups are relatively hydrophobic, enabling efficient capture of target proteins primarily through hydrophobic interactions. Simultaneously, target proteins are positively charged below their isoelectric point (pI) and may experience weak electrostatic attraction to any remaining unprotonated -COO⁻ groups.
Non-Specific Adsorption May Be Higher: Increased surface hydrophobicity can lead to higher non-specific binding of other hydrophobic impurities.
pH ≈ pKa (~4.5-5.0):
Approximately 50% of carboxyl groups are protonated (-COOH), 50% are deprotonated (-COO⁻).
Binding behavior is complex, involving a mixture of hydrophobic and electrostatic interactions.
High pH (Alkaline, > pKa):
Carboxyl groups are highly deprotonated (-COO⁻), resulting in a strongly negatively charged surface.
Very Low Hydrophobic Binding Capacity: The highly hydrophilic, charged surface is unfavorable for hydrophobic interactions.
Ion Exchange Capability Emerges: The negatively charged surface can be used for anion exchange to bind positively charged substances (though this is not the primary design purpose of most carboxyl beads).
Low Non-Specific Adsorption: Strong hydrophilicity and negative charge effectively reduce non-specific binding (often utilized in blocking or wash steps).
Elution Conditions: Under alkaline conditions (high pH), target proteins are readily eluted due to electrostatic repulsion (if the protein is negatively charged) or weakened hydrophobic interactions caused by increased hydrophilicity. Low pH elution is also common, achieved by protonating carboxyl groups (reducing hydrophobic interactions) and protonating the target protein (introducing electrostatic repulsion).
Optimal Range:
Binding: Typically performed under acidic pH (pH 4.0 – 5.5) to maximize carboxyl protonation and utilize hydrophobic interactions for target protein binding. Common buffers include sodium acetate.
Washing: Often done at near-neutral or weakly alkaline pH to minimize non-specific binding.
Elution: Commonly achieved using low pH buffers (e.g., pH 2.5 – 3.5, Glycine-HCl) or high pH buffers (e.g., pH 8 – 11, Tris or Glycine-NaOH).
Summary Comparison Table
| Characteristic | Silica hydroxyl Magnetic Beads (for Nucleic Acids) | Carboxyl Magnetic Beads (for Proteins/Antibodies) |
| Primary Binding Force | Salt Bridge (Cation-mediated electrostatic attraction) | Hydrophobic Interactions (+ Hydrogen Bonding) |
| Key Functional Group | -Si-OH / -Si-O⁻ | -COOH / -COO⁻ |
| Optimal Binding pH | Alkaline (pH 8.0 – 9.5) | Acidic (pH 4.0 – 5.5) |
| Effect of Low pH | Very Weak Binding: Groups protonated (-Si-OH), insufficient charge or positive charge | Strong Binding: Carboxyls protonated (-COOH), high hydrophobicity |
| Effect of High pH | Strong Binding: Groups deprotonated (-Si-O⁻), high negative charge density Risk: Dissolution (>pH 10-11) | Very Weak Binding: Carboxyls deprotonated (-COO⁻), highly hydrophilic/charged Use: Elution, reducing non-specific binding |
| Critical pH Threshold | Dissolution Threshold: pH >10-11 (especially with heat) | pKa Value: ~4.5-5.0 (binding/elution transition point) |
| Typical Buffers | Tris, Sodium Acetate with high chaotropic salts (pH~8-9) | Sodium Acetate (pH~4-5.5) for binding; Low/High pH buffers for elution |
Conclusion:
pH is a core parameter controlling the function of both Silica hydroxyl and carboxyl magnetic beads. Choosing the wrong pH can directly lead to binding failure, difficult elution, high non-specific binding, or bead degradation.
Opposite Direction of Effect: Silica hydroxyl beads require alkaline pH for efficient binding (nucleic acids), whereas carboxyl beads require acidic pH for efficient binding (proteins/antibodies).
Follow Protocols: Strictly adhere to the recommended pH ranges for buffers as specified in the product manual for the specific beads being used. Deviation from the recommended pH is one of the most common causes of experimental failure.
Understand the Mechanism: Understanding how pH affects the charge and properties of the bead surface groups aids in making informed decisions when optimizing experiments or troubleshooting problems.
Therefore, precise control of buffer pH during operation is crucial for ensuring that Silica hydroxyl or carboxyl magnetic beads perform as expected. The importance of this cannot be overemphasized.
Supplier
Shanghai Lingjun Biotechnology Co., Ltd. was established in 2016 which is a professional manufacturer of biomagnetic materials and nucleic acid extraction reagents.
We have rich experience in nucleic acid extraction and purification, protein purification, cell separation, chemiluminescence, and other technical fields.
Our products are widely used in many fields, such as medical testing, genetic testing, university research, genetic breeding, and so on. We not only provide products but also can undertake OEM, ODM, and other needs. If you have a related need, please feel free to contact us .