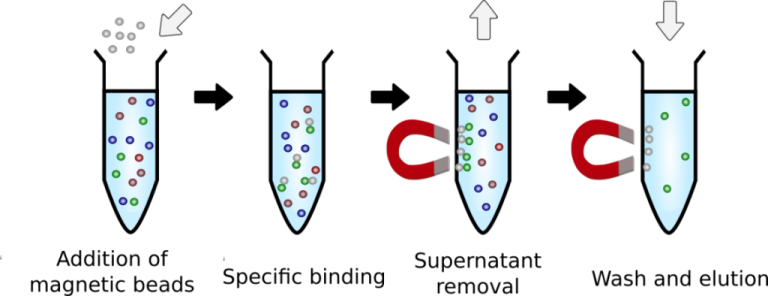
Professional Manufacturer of Biomagnetic Beads

Mechanism of action of sodium citrate and sodium acetate in magnetic bead synthesis
In the synthesis process of magnetic beads (especially magnetite Fe₃O₄ beads), particularly in the coprecipitation method, sodium citrate and sodium acetate play distinct but crucial roles. Their mechanisms primarily revolve around controlling particle nucleation and growth, preventing agglomeration, stabilizing dispersion, and regulating particle morphology and size. Below is a detailed explanation of their respective mechanisms:
I. Sodium Citrate (Na₃C₆H₅O₇)
Sodium citrate primarily acts as a surfactant/stabilizer, chelating agent, and charge regulator in magnetic bead synthesis.
Surface Modification and Stabilization (Primary Role):
Adsorption and Coordination: The citrate anion (C₆H₅O₇³⁻) possesses three carboxylate groups (-COO⁻). These groups have strong chelating ability and form robust complexes with Fe²⁺/Fe³⁺ ions on the surface of newly formed Fe₃O₄ nanoparticles, firmly adsorbing onto the particle surface.
Steric Hindrance Stabilization: The adsorbed citrate molecules extend outward, forming a hydrophilic “shell” or “protective layer.” When particles approach each other, this shell creates a physical barrier, preventing direct contact and agglomeration (steric stabilization mechanism).
Electrostatic Stabilization: The adsorbed citrate anions impart a negative charge to the magnetic bead surface (increasing the negative Zeta potential). The resulting electrostatic repulsion between like-charged particles further prevents agglomeration (electrostatic stabilization mechanism). The combined action of steric hindrance and electrostatic repulsion significantly enhances the colloidal stability of the beads in aqueous solution.
Controlling Particle Size and Morphology:
Growth Limitation: By tightly coating the particle surface, sodium citrate limits the exposure of active surface sites, thereby slowing down Ostwald ripening (dissolution of small particles and growth of larger ones) and particle fusion. This helps achieve smaller nanoparticles with a more uniform size distribution.
Influencing Nucleation: Citrate anions can form soluble complexes with Fe²⁺/Fe³⁺ ions in solution (see Chelation below), somewhat reducing the concentration of free metal ions. This may indirectly affect the nucleation rate and the final number of particles.
Chelation:
Citrate can form stable, water-soluble complexes with Fe²⁺ and Fe³⁺ ions in solution (e.g., [Fe(C₆H₅O₇)]⁻, [Fe(C₆H₅O₇)]⁰, etc.). This serves two purposes:
Buffering Metal Ion Concentration: Chelation lowers the instantaneous concentration of free Fe²⁺/Fe³⁺ ions, making the precipitation reaction (nucleation and growth) more gradual and controllable.
Reducing Impurity Formation: By controlling free ion concentration, it favors the formation of a purer, better-crystallized Fe₃O₄ phase and reduces the generation of other iron oxide impurities (e.g., goethite, hematite).
Providing a Basis for Biocompatibility (Subsequent Applications):
Citrate-modified beads have surfaces rich in carboxyl groups (-COOH). These groups are highly hydrophilic and biocompatible. They also readily allow covalent conjugation with biomolecules (e.g., antibodies, proteins, nucleic acids) via reactions like amidation, laying the foundation for biomedical applications (e.g., separation, detection, targeted drug delivery).
Summary of Sodium Citrate’s Mechanism: Achieves stable dispersion via strong adsorption/chelation on the particle surface, providing steric hindrance and electrostatic repulsion. Controls particle size, morphology, and crystallinity by chelating metal ions and limiting surface growth. Simultaneously provides reactive groups for subsequent biofunctionalization.
II. Sodium Acetate (CH₃COONa)
Sodium acetate primarily acts as an alkaline source, buffer, and structure-directing agent in magnetic bead synthesis (especially in hydrothermal or solvothermal methods).
Providing Alkaline Conditions (Core Function):
Fe₃O₄ formation requires alkaline conditions. Sodium acetate hydrolyzes to produce OH⁻ ions:
CH₃COO⁻ + H₂O ⇌ CH₃COOH + OH⁻
This hydrolysis reaction is relatively mild, providing a sustained, controllable alkaline environment for the coprecipitation of Fe²⁺ and Fe³⁺ ions. This is crucial for generating Fe₃O₄ instead of other iron oxides (e.g., α-Fe₂O₃, γ-Fe₂O₃). Insufficient alkalinity tends to produce non-magnetic iron hydroxide colloids.
Buffering Action:
The acetate ion (CH₃COO⁻) and its conjugate acid, acetic acid (CH₃COOH), form a buffer pair. During the reaction (especially under high-temperature hydrothermal/solvothermal conditions), it effectively buffers the pH of the system, preventing drastic local pH fluctuations.
A stable pH environment is essential for controlling the uniformity of the precipitation reaction, obtaining well-crystallized Fe₃O₄ crystals, and ensuring a uniform particle size distribution. Sharp pH changes can lead to explosive nucleation or amorphous precipitate formation, resulting in uneven particle sizes or agglomeration.
Structure-Directing and Morphology Control:
At high concentrations or under specific reaction conditions (e.g., hydrothermal methods), acetate ions can adsorb onto specific crystal facets of Fe₃O₄, altering the relative surface energies of different facets, thereby influencing anisotropic crystal growth.
This adsorption can guide particles to form specific morphologies, such as spheres, cubes, octahedrons, or even flower-like structures. Sodium acetate is a commonly used additive for achieving morphology-controlled synthesis.
Promoting Crystallization:
Under the high-temperature and high-pressure environment of hydrothermal/solvothermal reactions, the alkaline buffering environment provided by sodium acetate facilitates the dissolution-recrystallization process of Fe₃O₄ crystals. This promotes the dissolution of small particles and their recrystallization onto larger ones, enhancing the crystallinity of the final product.
Mild Reducing Atmosphere (Secondary Role):
At high temperatures, acetate or acetic acid may decompose, producing small amounts of reducing gases (e.g., carbon monoxide or hydrogen) or weakly reducing intermediates. This helps maintain the stable presence of Fe²⁺ in the reaction system (preventing its excessive oxidation to Fe³⁺), ensuring the formation of Fe₃O₄ close to its stoichiometric ratio (Fe²⁺:Fe³⁺ = 1:2).
Summary of Sodium Acetate’s Mechanism: Creates a stable, suitable alkaline environment for Fe₃O₄ formation and crystallization by hydrolyzing to provide sustained, controllable OH⁻ and buffering pH. Under specific conditions, it achieves morphology control by adsorbing and regulating facet growth. It may also exhibit weak reducing properties to maintain the Fe²⁺ oxidation state.
Key Differences and Synergistic Effects
Core Functions Differ: The core function of sodium citrate is surface modification and stabilization/dispersion, while sodium acetate’s core function is providing and maintaining the required alkaline reaction environment.
Stage of Action: Sodium citrate primarily acts immediately after particle formation (stabilizing nascent particles) and influences growth. Sodium acetate acts throughout the precipitation reaction (providing OH⁻ and buffering pH), being crucial especially during initial nucleation and crystal growth.
Synergistic Effect: In optimized processes, they are often used together:
Sodium acetate ensures the generation of well-crystallized Fe₃O₄ primary particles at a suitable and stable pH.
Sodium citrate rapidly coats these primary particles, preventing their agglomeration into large precipitates and controlling the size and dispersity of the final nanoparticles.
The chelating action of sodium citrate also complements sodium acetate in maintaining system stability.
In summary, in magnetic bead synthesis:
Sodium citrate is an excellent surface stabilizer, dispersant, and size control agent, paving the way for biological applications.
Sodium acetate is a key alkaline source, pH buffer, and morphology control agent, ensuring the formation of high-quality Fe₃O₄ crystals.
Understanding their distinct mechanisms is essential for optimizing the formulation and process parameters (e.g., concentration, temperature, addition method) in magnetic bead synthesis to obtain magnetic nanoparticles with desired properties (small size, narrow distribution, stable dispersion, good crystallinity, controllable morphology).
Supplier
Shanghai Lingjun Biotechnology Co., Ltd. was established in 2016 which is a professional manufacturer of biomagnetic materials and nucleic acid extraction reagents.
We have rich experience in nucleic acid extraction and purification, protein purification, cell separation, chemiluminescence, and other technical fields.
Our products are widely used in many fields, such as medical testing, genetic testing, university research, genetic breeding, and so on. We not only provide products but also can undertake OEM, ODM, and other needs. If you have a related need, please feel free to contact us .