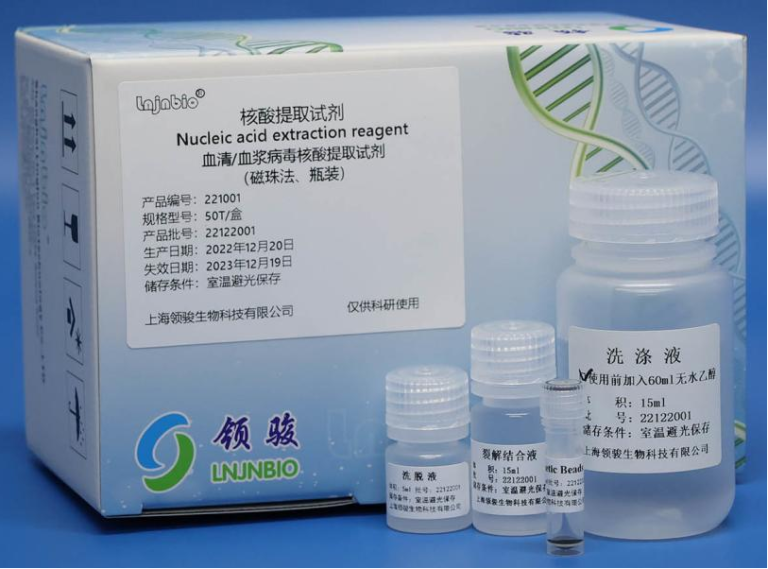
Professional Manufacturer of Biomagnetic Beads

The role of ferric chloride, ethylene glycol and polyethylene glycol in magnetic bead synthesis
In the solvothermal synthesis (e.g., hydrothermal or polyol methods) of magnetic beads (especially Fe₃O₄ beads), ferric chloride (FeCl₃), ethylene glycol (EG), and polyethylene glycol (PEG) work synergistically to regulate the nucleation, growth, morphology, and stability of the beads. Below is a detailed explanation of their mechanisms:
I. Role of Ferric Chloride (FeCl₃)
Core Function: Iron Source (Supplies Fe³⁺ Ions)
Providing Reaction Precursor
FeCl₃ dissociates in solution to release Fe³⁺ ions, serving as the primary iron source for Fe₃O₄ formation.
In reductive environments (e.g., ethylene glycol systems), Fe³⁺ is partially reduced to Fe²⁺, ultimately forming Fe₃O₄ (Fe²⁺Fe³⁺₂O₄).
Hydrolysis Control (Requires Caution)
Fe³⁺ readily hydrolyzes to form iron oxyhydroxides (e.g., FeOOH), which may interfere with pure-phase Fe₃O₄ formation.
Hydrolysis must be suppressed via alkaline conditions (e.g., adding sodium acetate) or reducing agents (e.g., ethylene glycol) to ensure Fe₃O₄ generation.
II. Role of Ethylene Glycol (HO-CH₂-CH₂-OH)
Core Functions: Solvent, Reducing Agent, Surfactant
Reducing Agent (Critical Role)
At high temperatures (>150°C), ethylene glycol is oxidized to glyoxal (OHC-CHO) or acetic acid, reducing Fe³⁺ to Fe²⁺:
2Fe3++HOCH2CH2OH→2Fe2++OHC−CHO+2H+
Maintains the Fe²⁺/Fe³⁺ ≈ 1:2 ratio, ensuring formation of magnetic Fe₃O₄ instead of Fe₂O₃.
High-Boiling-Point Solvent
High boiling point (197°C) enables solvothermal/hydrothermal reactions (ambient/high-pressure, high-temperature conditions), facilitating crystal growth.
Surface Modification and Morphology Control
Hydroxyl groups (-OH) of ethylene glycol adsorb onto Fe₃O₄ surfaces, suppressing agglomeration via steric hindrance.
Modulates particle growth kinetics, promoting monodisperse spherical or cubic particles.
III. Role of Polyethylene Glycol (PEG, H-(O-CH₂-CH₂)_n-OH)
Core Function: Polymeric Surfactant (Dispersant, Morphology Director)
Steric Stabilizer
PEG chains adsorb onto particle surfaces, forming a hydrophilic protective layer that physically blocks particle agglomeration.
Significantly enhances long-term colloidal stability (especially in aqueous media).
plaintext
[Fe₃O₄ Core] ← PEG Chains (Hydrophilic Shell) → Barrier against particle approach
Morphology and Size Control
Chain length varies with molecular weight (e.g., PEG-2000, PEG-6000):
Short-chain PEG (low MW): Yields small particles (5-20 nm).
Long-chain PEG (high MW): Promotes self-assembly into complex structures (e.g., flower-like, chain-like).
Selective adsorption onto crystal facets guides anisotropic growth (e.g., cubes, octahedrons).
Enhanced Biocompatibility
PEG-coated beads feature ether (-O-) and hydroxyl (-OH) groups, providing:
Low protein adsorption → Reduced non-specific binding
High hydrophilicity → Ideal for biomedical applications (e.g., MRI contrast agents, drug carriers).
Synergistic Mechanism of the Trio
Initial Reaction Stage
FeCl₃ dissolves in ethylene glycol, forming Fe³⁺-EG complexes.
Ethylene glycol reduces Fe³⁺ to Fe²⁺ at high temperatures, while PEG adsorbs onto nascent nuclei.
Crystal Growth Stage
PEG controls growth direction/size; ethylene glycol maintains reducing environment.
Fe³⁺/Fe²⁺ coprecipitate as Fe₃O₄ under alkaline conditions (provided by sodium acetate, etc.).
Stability Assurance
PEG and ethylene glycol jointly form a dual protective layer (steric hindrance + electrostatic repulsion), preventing agglomeration.
Typical Synthesis Workflow (Solvothermal Method)
Key Differences and Summary
| Reagent | Core Function(s) | Unique Contribution |
| FeCl₃ | Iron source (Fe³⁺) | Reaction precursor |
| Ethylene Glycol | Solvent + Reducing agent + Dispersant | Reduces Fe³⁺→Fe²⁺, controls morphology |
| PEG | Polymeric surfactant | Steric stabilization, biocompatibility enhancement |
By adjusting ratios of these components and reaction conditions (temperature, time), bead size (5-200 nm), morphology (spheres, cubes, flowers), and magnetic responsiveness can be precisely engineered.
Supplier
Shanghai Lingjun Biotechnology Co., Ltd. was established in 2016 which is a professional manufacturer of biomagnetic materials and nucleic acid extraction reagents.
We have rich experience in nucleic acid extraction and purification, protein purification, cell separation, chemiluminescence, and other technical fields.
Our products are widely used in many fields, such as medical testing, genetic testing, university research, genetic breeding, and so on. We not only provide products but also can undertake OEM, ODM, and other needs. If you have a related need, please feel free to contact us .